(562) 256-9929
3736 ATLANTIC AVENUE
LONG BEACH, CA 90807
Injectables:
Dermal Fillers
Care by Board-Certified Dermatologists
Comprehensive Dermatology of Long Beach
Comprehensive Dermatology of Long Beach: Plump & Define with Hyaluronic Acid Fillers
Age-defying beauty meets the artistry of hyaluronic acid fillers. As time gracefully advances, our faces undergo a natural reduction in youthful volume, attributed to the diminishing production of hyaluronic acid—the essential cushioning and moisture retention agent in our skin, joints, nerves, hair, and eyes. Embrace the transformative power of our premier hyaluronic acid fillers: the Restylane® Collection of Fillers, the Juvederm® Collection of Fillers, the Revance RHA® Collection of Fillers, and Sculptra®. Safely and easily minimize wrinkles, restore facial fullness, and unlock a rejuvenated appearance with results lasting between 6 to 12 months. Trust in the FDA-approved safety of our fillers, harnessing the power of your body's natural processes. Rediscover your youthful radiance with us.
Schedule your appointment today and embark on a journey to timeless beauty. Your transformation awaits!
Comprehensive Dermatology of Long Beach:
Restylane®, JUVÉDERM® & Revance RHA® Collections of fillers - Rediscover youthful volume
At Comprehensive Dermatology of Long Beach, we offer a wide range of dermal fillers, including popular brands like Restylane®, Juvéderm®, Skinvive™ by Juvéderm®, and the Revance RHA® collections, to help you achieve your aesthetic goals. Dermal fillers are versatile injectable treatments that can address various concerns, such as smoothing fine lines and wrinkles, restoring lost volume, and enhancing facial contours.
Restylane and Juvéderm are composed of hyaluronic acid, a naturally occurring substance in the body that attracts and retains moisture, resulting in plumper, more hydrated skin. The Revance RHA collections feature resilient hyaluronic acid (RHA) technology, designed to mimic the natural movement and flexibility of facial tissue for natural-looking results.
Whether you desire subtle enhancement or dramatic rejuvenation, our experienced practitioners will create a customized treatment plan tailored to your unique needs and desired outcome. With dermal fillers at Comprehensive Dermatology of Long Beach, you can enjoy smoother, more youthful-looking skin and enhanced facial harmony.
Schedule a consultation today to discover how dermal fillers can help you achieve your aesthetic goals and regain confidence in your appearance.
Restylane® Collection Before & After


Actual patient. Results may vary. Treated with 1.5 mL Restylane® Lyft in each cheek, and 1.5 mL Restylane® Lyft in each nasolabial fold. Two weeks after treatment.
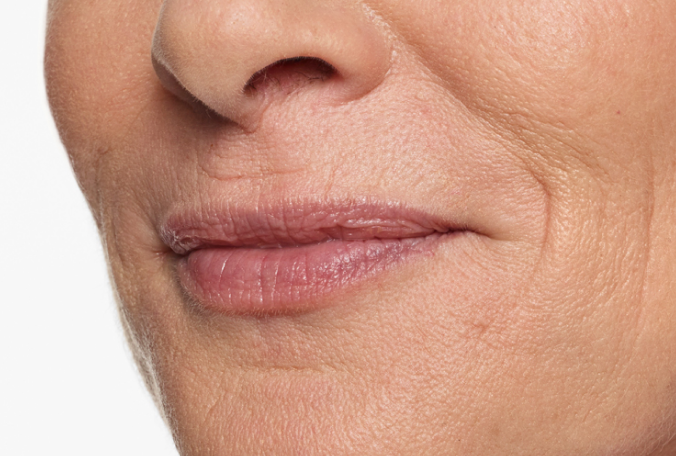
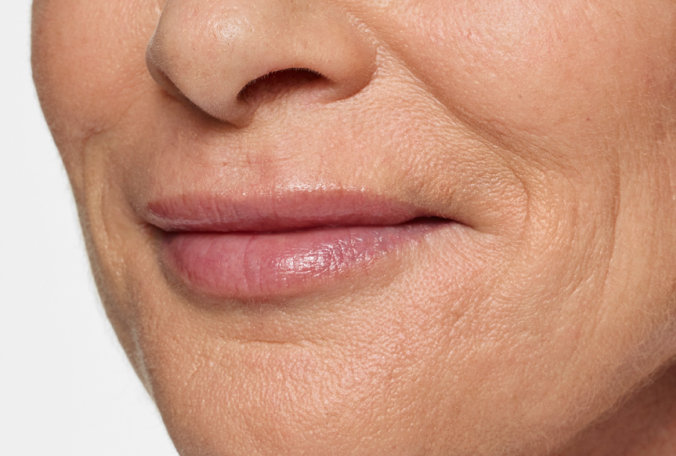
Actual patient. Results may vary. Treated with 2.3 mL of Restylane Silk in the lips and perioral (lip) lines around the mouth. Ten weeks after treatment.
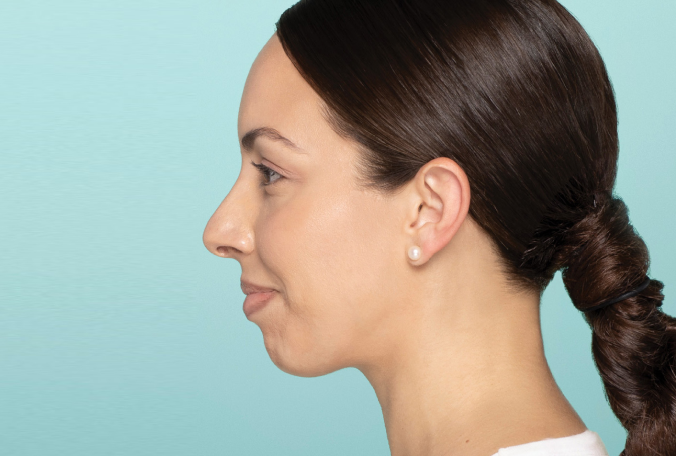
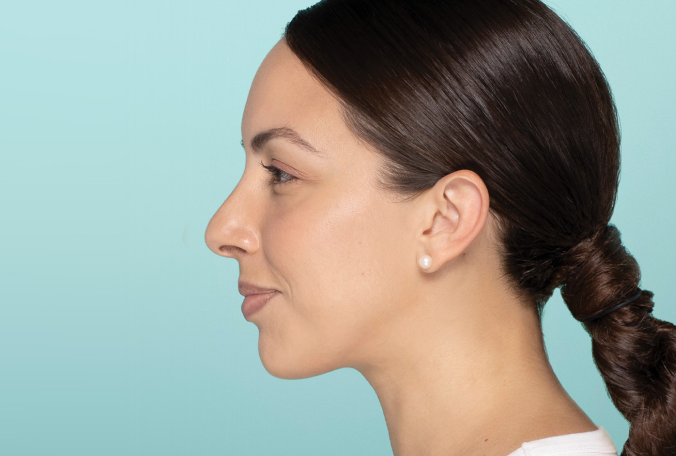
Actual patient. Results may vary. Treated with 2.6 mL of Restylane® Defyne in the chin. One week after treatment.
JUVÉDERM® Collection Before & After
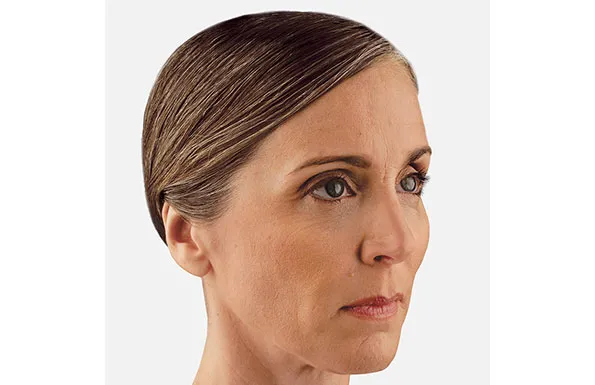
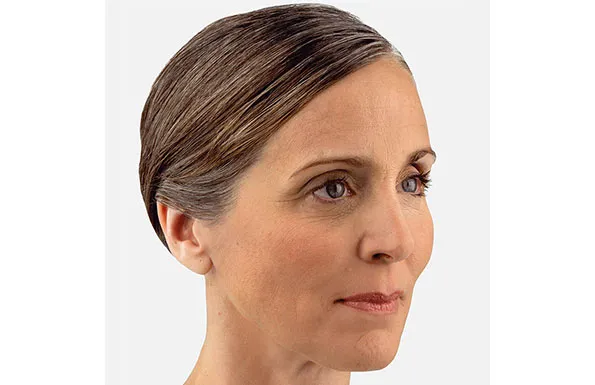
Actual patient. Results may vary. Unretouched photos taken before treatment and 1 month after treatment with 3.5 mL of JUVÉDERM® VOLUMA® XC in the cheeks.


Real patient treated with SKINVIVE™ by JUVÉDERM®. Actual results may vary.
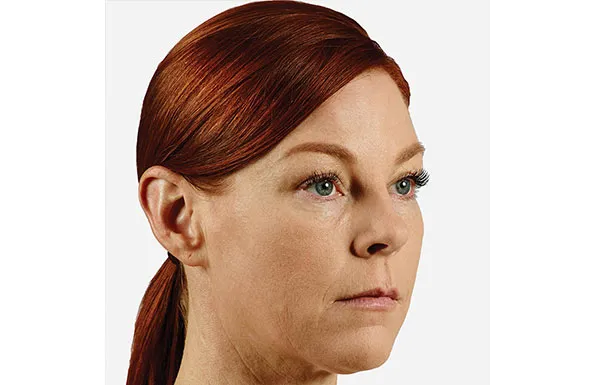
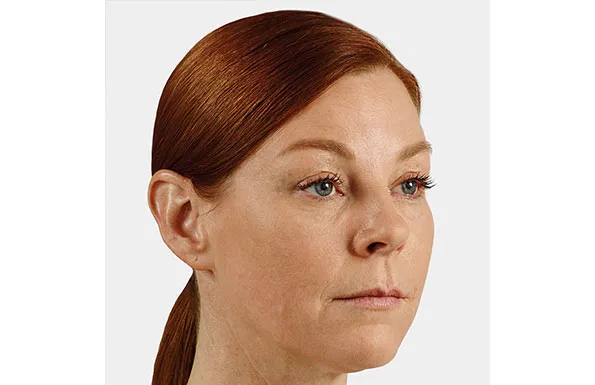
Actual patient. Results may vary. Unretouched photos taken before treatment and 1 month after treatment with 2.6 mL of JUVÉDERM® VOLUMA® XC in the cheeks.
RHA® Collection Before & After


Actual patient. Results may vary. TREATMENT AREAS: RHA® 4 1.4 mL for direct nasolabial fold correction, RHA® 4 1.6 mL for indirect nasolabial fold correction, and RHA® 4 0.6 mL in oral commissures.


Actual patient. Results may vary. TREATMENT AREAS: RHA Redensity™ 0.7 mL in perioral rhytids; RHA® 2 1.5 mL in radial cheek lines; and RHA® 3 0.8 mL for direct nasolabial fold correction, 0.2 mL for indirect nasolabial fold correction
Comprehensive Dermatology of Long Beach:
Revitalize Your Youth with Sculptra® Facial Filler
Experience lasting facial rejuvenation with Sculptra at Comprehensive Dermatology of Long Beach. FDA-approved and made with poly-L-lactic acid, this unique facial filler stimulates natural collagen production, ensuring natural-looking results that last up to 2 years.
Crafted with poly-L-lactic acid, Sculptra is a synthetic, biocompatible, and biodegradable substance widely used in reconstructive surgery for dissolvable stitches and soft tissue implants. FDA-approved for treating deep folds, marionette lines, and chin wrinkles, Sculptra stands as an exceptional anti-aging filler capable of delivering dramatic results for up to 2 years.
Discover the transformative power of Sculptra as it addresses deep folds, marionette lines, and chin wrinkles. This exceptional anti-aging filler not only ensures remarkable results but also boasts a unique approach by actively stimulating your body's natural collagen production. At Comprehensive Dermatology of Long Beach, our expert
practitioners are dedicated to providing you with a revitalized and natural-looking appearance that lasts, allowing you to embrace your timeless beauty for up to 2 years. Schedule your consultation today and let Sculptra redefine your journey to youthful radiance.
Slide title
Write your caption hereButton

Slide title
Write your caption hereButton
-
Important Safety Information - Restylane® Collection of Fillers
Important Safety Information
The Restylane family of products are indicated for patients over the age of 21, and includes Restylane®, Restylane-L®, Restylane® Lyft with Lidocaine, Restylane® Silk, Restylane® Kysse, Restylane® Refyne, Restylane® Defyne, Restylane® Contour, and Restylane® Eyelight.
Approved Uses
Restylane® and Restylane-L® are for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. Restylane® and Restylane-L® are also indicated for injection into the lips.
Restylane® Lyft with Lidocaine is for deep implantation into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds and for cheek augmentation and for the correction of age-related midface contour deficiencies. Restylane® Lyft with Lidocaine is also indicated for injection into the dorsal hand to correct volume loss.
Restylane® Silk is for lip augmentation and for correction of perioral wrinkles.
Restylane® Kysse is for lip augmentation and for correction of upper perioral wrinkles.
Restylane® Refyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds.
Restylane® Defyne is for mid-to-deep injection into the facial tissue for the correction of moderate to severe deep facial wrinkles and folds, such as nasolabial folds. Restylane® Defyne is also indicated for injection into the mid-to-deep dermis (subcutaneous and/or supraperiosteal) for augmentation of the chin region to improve the chin profile in patients with mild to moderate chin retrusion.
Restylane® Contour is for cheek augmentation and for the correction of midface contour deficiencies.
Restylane® Eyelight is for the improvement of infraorbital hollowing.
Do not use if you have severe allergies with a history of severe reactions (anaphylaxis), are allergic to lidocaine or gram-positive bacterial proteins used to make hyaluronic acid, prone to bleeding, or have a bleeding disorder. The safety of use while pregnant or breastfeeding has not been studied. Tell your doctor if you have a history of scarring or pigmentation disorders as these side effects can occur with hyaluronic acid fillers. Tell your doctor if you are planning other cosmetic treatments (i.e., lasers and chemical peels) as there is a possible risk of inflammation at the injection site.
Tell your doctor if you’re taking medications that lower your body’s immune response or affect bleeding, such as aspirin or warfarin, as these medications may increase the risk of bruising or bleeding at the gel injection site. Using these products on gel injection sites with skin sores, pimples, rashes, hives, cysts, or infections should be postponed until healing is complete.
The most common side effects are swelling, redness, pain, bruising, headache, tenderness, lump formation, itching at the injection site, and impaired hand function. Delayed-onset inflammation near the site of dermal filler injections is one of the known adverse events associated with dermal fillers, and cases have been reported to occur at the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. Typically, the reported inflammation was responsive to treatment or resolved on its own. Serious but rare side effects include delayed onset infections, recurrence of herpetic eruptions, and superficial necrosis at the injection site. The risk of unintentional injection into a blood vessel is small but can occur and could result in serious complications, which may be permanent including, vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. As with all skin injection procedures, there is a risk of infection.
To report a side effect with any Restylane® product, please call Galderma Laboratories, L.P. at 1-855-425-8722.
To learn more about serious but rare side effects and full Important Safety Information, visit www.RestylaneUSA.com.
US-RES-2300327
-
Important Safety Information - JUVÉDERM® Collection of Fillers
MPORTANT SAFETY INFORMATION
Do not use these products if you have a history of multiple severe allergies or severe allergic reactions (anaphylaxis), if you are allergic to lidocaine or the Gram-positive bacterial proteins used in these products, or if you have had previous allergic reactions to hyaluronic acid fillers.
APPROVED USES
JUVÉDERM® VOLUX® XC injectable gel is for deep injection to improve moderate to severe loss of jawline definition in adults over the age of 21.
JUVÉDERM® VOLUMA® XC injectable gel is for deep injection in the cheek area to correct age-related volume loss and for augmentation of the chin region to improve the chin profile in adults over 21.
JUVÉDERM® VOLLURE® XC, JUVÉDERM® Ultra Plus XC, and JUVÉDERM® Ultra XC injectable gels are for injection into the facial tissue for the correction of moderate to severe facial wrinkles and folds, such as nasolabial folds. JUVÉDERM® VOLLURE® XC injectable gel is for adults over 21.
JUVÉDERM® Ultra XC injectable gel is also for injection into the lips and perioral area for lip augmentation in adults over 21.
JUVÉDERM® VOLBELLA® XC injectable gel is for injection into the lips for lip augmentation and correction of perioral lines, and for injection into the undereye hollows to improve the appearance of undereye hollows in adults over the age of 21.
JUVÉDERM® Injectable Gel Fillers Important Safety Information
ARE THERE ANY REASONS WHY I SHOULD NOT RECEIVE ANY JUVÉDERM® FORMULATION?
Do not use these products if you have a history of multiple severe allergies or severe allergic reactions (anaphylaxis), if you are allergic to lidocaine or the Gram-positive bacterial proteins used in these products, or if you have had previous allergic reactions to hyaluronic acid fillers.
WHAT WARNINGS SHOULD MY DOCTOR ADVISE ME ABOUT?
One of the risks with using dermal fillers is the unintentional injection into a blood vessel. The chances of this happening are very small, but if it does happen, the complications can be serious and may be permanent. These complications, which have been reported for facial injections, can include vision abnormalities, blindness, stroke, temporary scabs, or permanent scarring of the skin. Most of these events are irreversible.
If you have changes in your vision, signs of a stroke (including sudden difficulty speaking, numbness or weakness in your face, arms or legs, difficulty walking, face drooping, severe headache, dizziness, or confusion), white appearance of the skin, or unusual pain during or shortly after treatment, you should notify your health care practitioner immediately.
The use of dermal fillers where skin sores, pimples, rashes, hives, cysts, or infections are present should be postponed, as this may delay healing or make skin problems worse.
The effectiveness of removal of any dermal filler has not been studied.
WHAT PRECAUTIONS SHOULD MY DOCTOR ADVISE ME ABOUT?
JUVÉDERM® VOLBELLA® XC should only be injected into undereye hollows by doctors who have completed the necessary training for this treatment area. To find a doctor, visit Juvederm.com/find-a-specialist. Doctors who complete the training will be listed with a symbol
The safety of these products for use during pregnancy or while breastfeeding has not been studied
The safety of JUVÉDERM® VOLUMA® XC has not been studied in patients under 35 years or over 65 years for cheek augmentation, or under 22 years and over 80 years for chin augmentation. The safety of JUVÉDERM® VOLUX® XC, JUVÉDERM® VOLLURE® XC and JUVÉDERM® VOLBELLA® XC has not been studied in patients under 22 years, and the safety of JUVÉDERM® Ultra Plus XC and JUVÉDERM® Ultra XC has not been studied in patients under 18 years
The safety and effectiveness of treatment with JUVÉDERM® products in anatomical regions outside of their approved uses have not been established in clinical studies
If you have a history of excessive scarring (thick, hard scars) or pigmentation disorders, treatment in these patients has not been studied and may result in additional scars or changes in pigmentation
If you are planning other procedures including laser treatments or a chemical peel, there is a possible risk of inflammation at the treatment site if these procedures are performed closely before or after JUVÉDERM® injectable gel treatment
Tell your doctor if you are on therapy used to reduce your body's natural defense system (such as steroids, chemotherapy, and medicines to treat autoimmune diseases, HIV, and AIDs), as these may increase your risk of infection; and medications that can prolong bleeding (such as aspirin, ibuprofen, or other blood thinners), as these may result in increased bruising or bleeding at the injection site.
Avoid applying makeup for 12 hours after treatment and minimize strenuous exercise, exposure to extensive sun or heat, and alcoholic beverages within the first 24 hours following treatment, as these may cause temporary redness, swelling, and/or itching at the injection site
JUVÉDERM® VOLUMA® XC was not studied in patients with significant loose skin of the chin, neck, or jaw
The effect of JUVÉDERM® VOLUMA® XC injection into the chin on facial hair growth has not been studied
Patients who experience skin injury near the site of JUVÉDERM® VOLUMA® XC injection may be at a higher risk for adverse events
Tell your doctor if you have already been injected with dermal fillers in the same area as the one(s) you are about to be treated for. This information helps your doctor decide when and whether you should get treatment
WHAT ARE POSSIBLE SIDE EFFECTS OF TREATMENT?
The most commonly reported side effects with JUVÉDERM® injectable gels were redness, swelling, pain, tenderness, firmness, lumps/bumps, bruising, discoloration, and itching. For JUVÉDERM® VOLBELLA® XC, dryness was also reported.
These side effects are consistent with other facial injection procedures and most will resolve within 30 days. Your doctor may choose to treat side effects persisting longer with antibiotics, steroids, or hyaluronidase (an enzyme that breaks down hyaluronic acid).
As with all skin injection procedures, there is a risk of infection.
To report a side effect with any product in the JUVÉDERM® Collection, please call the Allergan® Product Support Department at 1-877-345-5372. Please also visit Juvederm.com or talk to your doctor for more information.
Products in the JUVÉDERM® Collection are available only by a licensed physician or properly licensed practitioner.
-
Important Safety Information - RHA® Collection of Fillers
PLEASE SEE FULL DIRECTIONS FOR USE
RHA® Collection of Fillers
Indications: The RHA® Collection of resilient hyaluronic acid (HA) fillers includes RHA Redensity™, RHA® 2, RHA® 3 and RHA® 4. RHA Redensity™ is indicated for injection into the dermis and superficial dermis of the face, for the correction of moderate to severe dynamic perioral rhytids in adults 22 or older. RHA® 2 is indicated for injection into the mid-to-deep dermis for the correction of moderate to severe dynamic facial wrinkles and folds, such as nasolabial folds in adults 22 or older. RHA® 3 is indicated for injection into the mid-to-deep dermis for the correction of moderate to severe dynamic facial wrinkles and folds, such as nasolabial folds and is also indicated for injection into the vermillion body, vermillion border and oral commissure to achieve lip augmentation and lip fullness in adults 22 or older. RHA® 4 is indicated for injection in the deep dermis to superficial subcutaneous tissue for the correction of moderate to severe dynamic facial wrinkles and folds, such as nasolabial folds in adults 22 or older.
IMPORTANT SAFETY INFORMATION
Contraindications: Do not use in patients who have severe allergies, marked by a history of anaphylaxis or multiple severe allergies, or in patients with a history of allergies to gram-positive bacterial proteins or local anesthetics of the amide type, such as lidocaine. Do not use in patients with bleeding disorders.
Warnings: Do not inject into blood vessels. Introduction of these products into the vasculature may lead to embolization, occlusion of the vessels, ischemia, or infarction. Take extra care when injecting soft-tissue fillers; for example, inject the product slowly and apply the least amount of pressure necessary. Rare, but serious, adverse events associated with the intravascular injection of soft-tissue fillers in the face have been reported and include temporary or permanent vision impairment, blindness, cerebral ischemia or cerebral hemorrhage leading to stroke, skin necrosis, and damage to underlying facial structures. Immediately stop the injection if a patient exhibits any of the following symptoms: changes in vision, signs of a stroke, blanching of the skin, or unusual pain during or shortly after the procedure. Patients should receive prompt medical attention and, possibly, evaluation by an appropriate healthcare professional specialist should an intravascular injection occur. Product use at specific sites in which an active inflammatory process or infection is present should be deferred until the underlying process has been controlled.
Precautions: These products should only be used by healthcare professionals who have appropriate training, experience, and knowledge of facial anatomy.
Discuss the potential risks of soft-tissue injections with your patients prior to treatment and ensure that patients are aware of signs and symptoms of potential complications.
The safety and effectiveness for the treatment of anatomic regions other than the labeled indications have not been established in controlled U.S. clinical studies.
As with all transcutaneous procedures, dermal filler implantation carries a risk of infection. Standard precautions associated with injectable materials should be followed.
The safety for use in sites in the presence of other implants, during pregnancy, in breastfeeding females, and in patients with known susceptibility to keloid formation, hypertrophic scarring, and pigmentation disorders has not been studied.
Use with caution in patients on immunosuppressive therapy.
Patients who are using products that can prolong bleeding (such as thrombolytics, anticoagulants, or inhibitors of platelet aggregation) may experience increased bruising or bleeding at treatment sites.
Patients with a history of herpetic eruptions may experience reactivation of the herpes.
There is a possible risk of inflammation at the implant site if laser treatments or a chemical peel are performed after treatment.
Use as supplied. Modification or use of the product outside the Directions for Use may adversely impact the sterility, safety, homogeneity, or performance of the product. For single patient use. Do not reuse a syringe between two treatments and/or between two patients. Do not resterilize.
Adverse Events: The most commonly reported side effects were firmness, redness, tenderness, swelling, lumps/bumps, bruising, discoloration, pain and itching. Most of these events were mild or moderate and resolved within 14 days. Delayed-onset inflammation near the site of dermal filler injections is one of the known adverse events associated with dermal fillers. Cases of delayed-onset inflammation have been reported to occur at the dermal filler treatment site following viral or bacterial illnesses or infections, vaccinations, or dental procedures. Typically, the reported inflammation was responsive to treatment or resolved on its own. To report an adverse reaction with any RHA® product, please call Revance at (877) 373-8669.
RHA-P-002436.3
REFERENCES 1. RHA® Directions for Use. Nashville, TN: Revance Therapeutics, Inc, 2023. 2. Monheit G, Kaufman-Janette J, Joseph JH, Shamban A, Dover JS, Smith S. Dermatol Surg. 2020;46(12):1521-1529. 3. Kaufman-Janette J, Taylor SC, Cox SE, Weinkle SH, Smith S, Kinney BM. J Cosmet Dermatol. 2019;18(5):1244-1253. 4. Sundaram H, Shamban A, Schlessinger J, et al. Dermatol Surg. 2022;48(1):87-93. 5. Data on file. RDRE 2016—US Products, 2016. Newark, CA: Revance Therapeutics, Inc, 2016. 6. Faivre J, Gallet M, Tremblais E, Trévidic P, Bourdon F. Dermatol Surg. 2021;47(5):159-167. 7. Data on file. TEO-RHA-1501 Clinical Study Report. Newark, CA: Revance Therapeutics, Inc, 2019. 8. Mashburn J, Faivre J, Bourdon F. Evaluation of the impact of hyaluronic acid (HA) filler manufacturing technologies on HA chain degradation. Poster presented at: American Society for Dermatologic Surgery Virtual Annual Meeting; October 9-11, 2020.
-
Important Safety Information - Sculptra®
Important Safety Information
Indication: Sculptra® (injectable poly-L-lactic acid (PLLA-SCA)) is indicated for use in people with healthy immune systems for the correction of shallow to deep nasolabial fold contour deficiencies, fine lines and wrinkles in the cheek region, and other facial wrinkles.
Sculptra should not be used by people that are allergic to any ingredient of the product or have a history of keloid formation or hypertrophic scarring. Safety has not been established in patients who are pregnant, lactating, breastfeeding, or under 18 years of age.
Sculptra has unique injection requirements and should only be used by a trained healthcare practitioner. Contour deficiencies should not be overcorrected because they are expected to gradually improve after treatment.
Sculptra should not be injected into the blood vessels as it may cause vascular occlusion, infarction or embolic phenomena. Use at the site of skin sores, cysts, pimples, rashes, hives or infection should be postponed until healing is complete. Sculptra should not be injected into the red area (vermillion) of the lip or in the peri-orbital area.
The most common side effects after initial treatment include injection site swelling, tenderness, redness, pain, bruising, bleeding, itching and lumps. Other side effects may include small lumps under the skin that are sometimes noticeable when pressing on the treated area. Larger lumps, some with delayed onset with or without inflammation or skin discoloration, have also been reported.
Sculptra is available only through a licensed practitioner. View the complete Instructions for Use.
Instructions for Use
Sculptra — Instructions for Use
Patient Safety Information
Sculptra — Patient Safety Information
Choose Comprehensive Dermatology Of Long Beach for
Dermal Filler Treatments
Schedule Your Consultation Now!
Call us at (562) 256-9929 or click here to book online.
Get In Touch
Explore
Comprehensive Dermatology of Long Beach | All Rights Reserved
Privacy Policy | Site by Infinity Medical Marketing